I'm beyond glad that the test was pushed back because this unit is making me really nervous. We haven't done a information based unit in a while and it's mostly been all math so this is a slight change. I'm not that great at remembering rules, numbers, and etc so this is a rough unit for me.
Unit Summary:
The Wave Nature of Light
- Electronic structure: arrangement of electrons in an atom
- visible light: ROY G BIV from least to highest energy
- << electromagnetic spectrum
- higher amplitude and shorter wavelength= more energy
Wavelength formula:
H= 6.63*10^-34
Energy Formula:
Organization of Electrons:
- Principal Energy Level: n=1,2,3,4,5,6,7 (distance from nucleus)
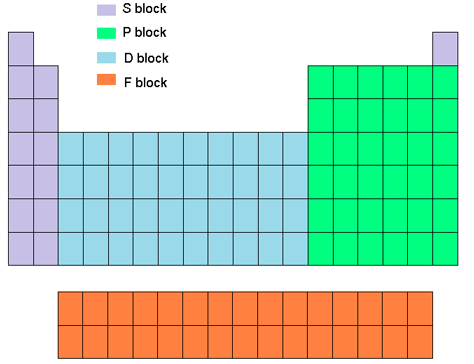
- Sublevel: s,p,d,f (different sections on table)
- Orbitals: s- 1, p-3, d-5, f-7; each orbital holds 2 electrons max
- Spin: Can have either an upward (+1/2) or downward (-1/2) spin
Rules for placing Electrons:
- Aufbau Principle: electrons enter orbitals lowest energy first
- Pauli Exclusion Principle; an orbital can only contain 2 electrons with an opposite spin
- Hund's Rule: within a sublevel, electrons must enter singly (positive first)
Energy Level Diagram for Sublevels: Electron Configurations:
- read from left to right
- Short cut with putting previous noble gas in brackets [ ]
- Exceptions where you remove one electron from the s sublevel and add to the p: Co, Cr, Mo, W, Ag, Au
Configuration of Ions: add or remove the appropriate amount of electrons from the highest principal energy level first
Quantum numbers
Links I used to help me study:
No comments:
Post a Comment