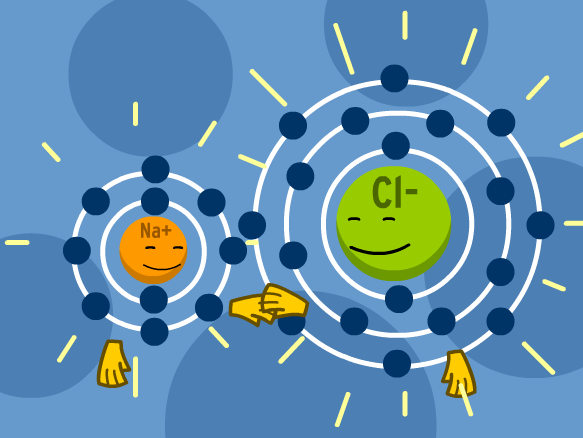
Links
Lewis Structures Practice --- Answers
This Link explains how to draw 3D Lewis structures and shows a few examples
Chemical Bonding Practice Multiple Choice Test
Formal Charge Khan Academy Video
Bond Polarity
Lewis Structures Khan Academy- this video showed a different way of drawing these bonds but I liked it a lot better because for simpler ones, it was very time saving
In the practice tests I thought this Lewis Structure was really hard so here is the correct structure for anyone else who had the same problem