I'm really nervous for the Unit Test tomorrow because I feel like we didn't get enough time and practice for this unit. One of the things I'm worried about is the acid base reactions because I don't recall learning that in class. I referred to the text book for this, but I also referred to outside links to learn more about it. As usual, I also did the Test Prep on schoology which usually helps me a lot.
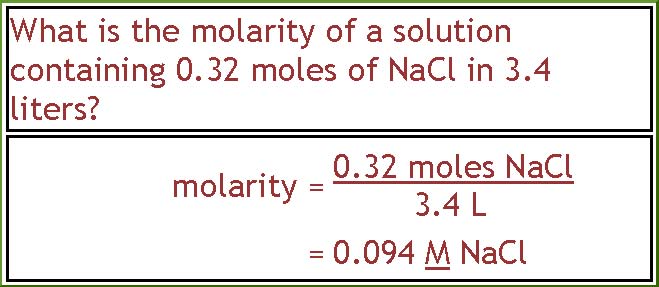
Ex-
Concentration of Ions:
Aqueous Solutions Summary
Solution: a homogeneous mixture
Solute: dissolved in the solvent
Solvent: present in the largest quantity in the solution
Solubility:
- Ionic Substances: anion and cation split if they are more attracted to the hydrogen and oxygen molecules in water
- Polar Substances: if the molecule has a similar structure to water, it will dissolve in water
- Substances that are insoluble in water: oil because it is nonpolar and therefore cannot dissolve in polar water. Chains of Carbons and Hydrogens cannot be dissolved in water
Solution Composition: amount of solute that can be dissolved in a solvent
- unsaturated: below saturation line
- saturated: on the saturation line
- supersaturated: above the saturation line
Factors that determine how fast a solute will dissolve:
- surface area: increased surface area to contact solvent
- stirring; increases surface area
- temperature: increased molecule activity

Ex-
Concentration of Ions:
Dilutions:
Aliquot: a portion of a larger whole, especially when dealing with a smaller sample of a mass concentration of a solution in chemistry
Using Molarities in Stoichiometry:
Neutralization Reactions/Acid Base Reactions:
(I still don't really get this part but it was in chapter 15 so I included it)
- when a strong acid and base react, they neutralize creating water and a salt
- you can use the molarity and volumes of the acid or base to find missing parts
Normality:
- equivalent of an acid: the amount of acid that can furnish 1 mol H+;ex: 1 mol HCl=1 equiz H+
- equivalent of a base: amount of that base that can furnish 1 mol H+
- example: 1/2 mol H2SO4=1 equiv H2SO4
This Link explains the Normality section but goes really into detail, however it does help in covering what is covered in the textbook.
Other Helpful Links:
Molarity in Stiochiometry: Video link
Practice:
acid base reaction: http://misterguch.brinkster.net/PRA048.pdf


No comments:
Post a Comment