I thought this unit was pretty simple in comparison to the last few units because this unit was more of an application of all the previous knowledge that we had and really the only new things we learned were the processes to solve certain problems. All the problems mostly consisted of the same basic process of starting with a balanced equation and went from converting to moles, to using the balanced equation ratio and then converting back to grams if necessary.
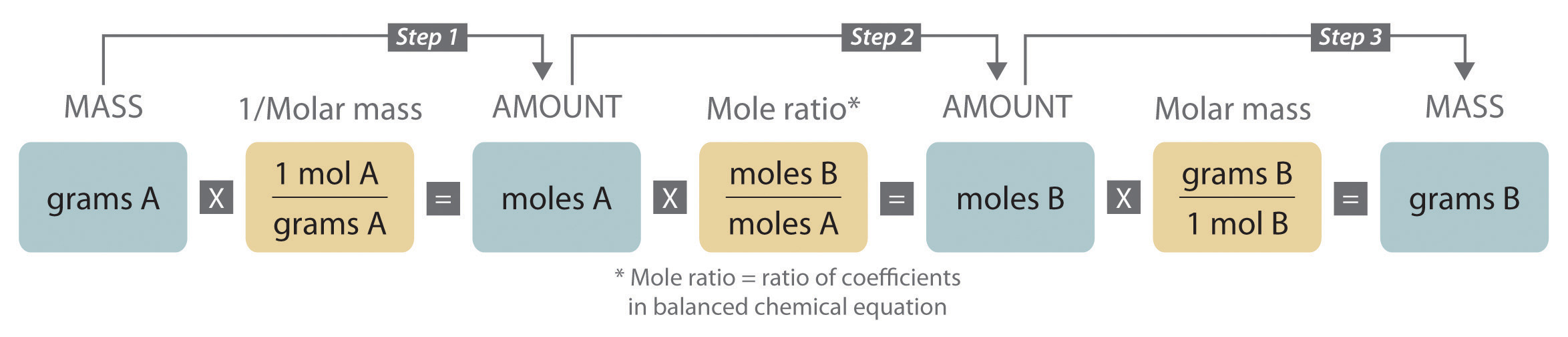
We can use the above process to find the mass or moles of any reactant or product in the balanced chemical equation if we are given a mass of a product or reactant to begin with.
Example: What number of moles of O2 would be produced by the decomposition of 13 grams of water.
More practice:
- http://www.chemistry.wustl.edu/~coursedev/Online%20tutorials/Plink/Stoichiometry/stoichset.htm
- http://science.widener.edu/svb/tutorial/genstoichiometrycsn7.html
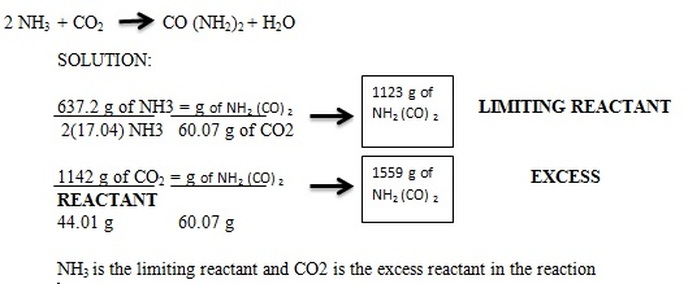
Limiting Reagent
Limiting reagent- the reactant that is completely used up when a reaction is run to completion
To find the limiting reagent we can run both reactions to find the amount of one product and the reactant that produces the least amount of product is the limiting reagent.
We can then use that to figure out how much product will actually be created (which will be the product created with the calculation of the limiting reagent)
Practice:
- http://www.chemistry.wustl.edu/~coursedev/Online%20tutorials/Plink/limreag/probsetlr.htm
- helpful link that explains the concept and gives examples: http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Limiting_Reagents
Percent Yield
Theoretical yield- the maximum amount of a given product that canbe formed when the limiting reagent is comopletely consumed
Percent yield- the actual yield of a product as the percentage of the theoretical yield
Practice:

I love how all your posts give lots of detail about each subject they cover, very well done!
ReplyDeleteI love how all your posts give lots of detail about each subject they cover, very well done!
ReplyDeleteThank you for making it more compact with all of our lessons in one post. Now I don't have to look at various posts to see the same information.
ReplyDeleteThese notes are awesome! I am studying for the test tonight, and I will definitely use these notes as a study tool. Shortened forms of the lesson are so helpful because they help me focus in on the concepts without having to spend a lot of time sifting through extra concepts I've already learned.
ReplyDelete